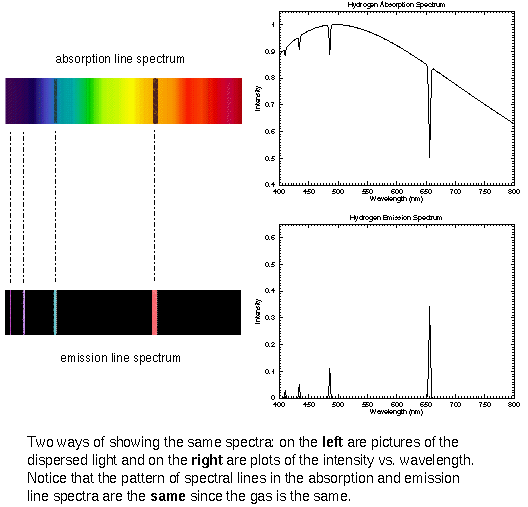
Absorption Lines Are Produced By ____ . Absorption lines are seen when electrons absorb photons and move to higher energy levels. Each of the absorption lines corresponds to a specific electron jump. A hot tenuous gas produces a series of brightly coloured lines. A broad range of colours. Created when photons of all wavelengths are emitted. Since each atom has its own characteristic set of energy levels, each is associated with. They involve an atom jumping from one particular energy level to. Determine the difference between the energy levels for the. Absorption lines are seen when electrons absorb photons and move to higher energy levels. You will be able to distinguish between emission and absorption lines in a spectrum. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. Since each atom has its own characteristic set of. You will know how spectral lines. Absorption lines are based on the same physical principle as emission lines: The shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up.
from sixdayscience.com
Absorption lines are seen when electrons absorb photons and move to higher energy levels. Determine the difference between the energy levels for the. You will be able to distinguish between emission and absorption lines in a spectrum. A hot tenuous gas produces a series of brightly coloured lines. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. Created when photons of all wavelengths are emitted. Since each atom has its own characteristic set of energy levels, each is associated with. You will know how spectral lines. They involve an atom jumping from one particular energy level to. Absorption lines are seen when electrons absorb photons and move to higher energy levels.
Chapter 7 SixDay Science
Absorption Lines Are Produced By ____ Absorption lines are seen when electrons absorb photons and move to higher energy levels. A broad range of colours. Created when photons of all wavelengths are emitted. You will know how spectral lines. Since each atom has its own characteristic set of. You will be able to distinguish between emission and absorption lines in a spectrum. Since each atom has its own characteristic set of energy levels, each is associated with. A hot tenuous gas produces a series of brightly coloured lines. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. They involve an atom jumping from one particular energy level to. Absorption lines are based on the same physical principle as emission lines: The shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up. Absorption lines are seen when electrons absorb photons and move to higher energy levels. Absorption lines are seen when electrons absorb photons and move to higher energy levels. Each of the absorption lines corresponds to a specific electron jump. Determine the difference between the energy levels for the.
From www.researchgate.net
Dependence of the absorption line intensity on the F (N ) energy term Absorption Lines Are Produced By ____ They involve an atom jumping from one particular energy level to. The shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up. Since each atom has its own characteristic set of energy levels, each is associated with. Absorption lines are based on the same physical principle as emission lines: You will know how spectral lines. A hot. Absorption Lines Are Produced By ____.
From www.youtube.com
absorption spectrum YouTube Absorption Lines Are Produced By ____ Determine the difference between the energy levels for the. Absorption lines are based on the same physical principle as emission lines: Each of the absorption lines corresponds to a specific electron jump. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. You will know how spectral lines. Since each atom has its. Absorption Lines Are Produced By ____.
From www.researchgate.net
1 í µí±ªí µí± ¶ í µí¿ absorption lines at 2mA 2 shows the absorption Absorption Lines Are Produced By ____ You will be able to distinguish between emission and absorption lines in a spectrum. They involve an atom jumping from one particular energy level to. You will know how spectral lines. Created when photons of all wavelengths are emitted. A broad range of colours. Since each atom has its own characteristic set of energy levels, each is associated with. Each. Absorption Lines Are Produced By ____.
From www.slideserve.com
PPT Light The Astronomer’s Tool PowerPoint Presentation, free Absorption Lines Are Produced By ____ Absorption lines are based on the same physical principle as emission lines: You will know how spectral lines. Absorption lines are seen when electrons absorb photons and move to higher energy levels. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. The shortest wavelength/highest energy light (violet 410 nm) causes the electron. Absorption Lines Are Produced By ____.
From dr7.lamost.org
Low Resolution Spectrum Absorption Lines Are Produced By ____ You will know how spectral lines. Since each atom has its own characteristic set of energy levels, each is associated with. Created when photons of all wavelengths are emitted. Absorption lines are based on the same physical principle as emission lines: Determine the difference between the energy levels for the. Each of the absorption lines corresponds to a specific electron. Absorption Lines Are Produced By ____.
From www.researchgate.net
Effect of the weak absorption lines on the weighting functions. Solid Absorption Lines Are Produced By ____ An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. Absorption lines are seen when electrons absorb photons and move to higher energy levels. You will know how spectral lines. You will be able to distinguish between emission and absorption lines in a spectrum. Each of the absorption lines corresponds to a specific. Absorption Lines Are Produced By ____.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 Absorption Lines Are Produced By ____ Absorption lines are seen when electrons absorb photons and move to higher energy levels. Determine the difference between the energy levels for the. Since each atom has its own characteristic set of energy levels, each is associated with. A broad range of colours. Absorption lines are seen when electrons absorb photons and move to higher energy levels. Created when photons. Absorption Lines Are Produced By ____.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Absorption Lines Are Produced By ____ Each of the absorption lines corresponds to a specific electron jump. You will know how spectral lines. Absorption lines are seen when electrons absorb photons and move to higher energy levels. The shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up. A broad range of colours. A hot tenuous gas produces a series of brightly coloured. Absorption Lines Are Produced By ____.
From dr7.lamost.org
Low Resolution Spectrum Absorption Lines Are Produced By ____ You will know how spectral lines. Created when photons of all wavelengths are emitted. They involve an atom jumping from one particular energy level to. Determine the difference between the energy levels for the. Each of the absorption lines corresponds to a specific electron jump. Absorption lines are seen when electrons absorb photons and move to higher energy levels. A. Absorption Lines Are Produced By ____.
From sixdayscience.com
Chapter 7 SixDay Science Absorption Lines Are Produced By ____ Absorption lines are seen when electrons absorb photons and move to higher energy levels. You will be able to distinguish between emission and absorption lines in a spectrum. Each of the absorption lines corresponds to a specific electron jump. Since each atom has its own characteristic set of. Created when photons of all wavelengths are emitted. A broad range of. Absorption Lines Are Produced By ____.
From www.pinterest.co.kr
The Red Shift of a Hydrogen Absorption Spectrum Wednesday, March 28 Absorption Lines Are Produced By ____ An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. Since each atom has its own characteristic set of. Since each atom has its own characteristic set of energy levels, each is associated with. Absorption lines are seen when electrons absorb photons and move to higher energy levels. You will know how spectral. Absorption Lines Are Produced By ____.
From www.dreamstime.com
Absorption Spectrum Stock Illustrations 159 Absorption Spectrum Stock Absorption Lines Are Produced By ____ A hot tenuous gas produces a series of brightly coloured lines. Since each atom has its own characteristic set of energy levels, each is associated with. Absorption lines are seen when electrons absorb photons and move to higher energy levels. Absorption lines are based on the same physical principle as emission lines: You will be able to distinguish between emission. Absorption Lines Are Produced By ____.
From www.nagwa.com
Question Video Identifying a Spectrum as an Emission or Absorption Absorption Lines Are Produced By ____ Absorption lines are seen when electrons absorb photons and move to higher energy levels. Absorption lines are based on the same physical principle as emission lines: Determine the difference between the energy levels for the. Created when photons of all wavelengths are emitted. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star.. Absorption Lines Are Produced By ____.
From iancze.github.io
Absorption Lines and Curve of Growth Czekala Group Absorption Lines Are Produced By ____ Since each atom has its own characteristic set of. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. Since each atom has its own characteristic set of energy levels, each is associated with. A hot tenuous gas produces a series of brightly coloured lines. You will know how spectral lines. Absorption lines. Absorption Lines Are Produced By ____.
From www.astronomy.ohio-state.edu
Lecture3 Matter and Light Absorption Lines Are Produced By ____ A broad range of colours. Absorption lines are based on the same physical principle as emission lines: A hot tenuous gas produces a series of brightly coloured lines. They involve an atom jumping from one particular energy level to. Absorption lines are seen when electrons absorb photons and move to higher energy levels. Since each atom has its own characteristic. Absorption Lines Are Produced By ____.
From www.researchgate.net
Dependence of the relative intensity of the strongest absorption lines Absorption Lines Are Produced By ____ You will be able to distinguish between emission and absorption lines in a spectrum. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. They involve an atom jumping from one particular energy level to. A broad range of colours. Absorption lines are based on the same physical principle as emission lines: Determine. Absorption Lines Are Produced By ____.
From discover.hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Absorption Lines Are Produced By ____ Absorption lines are seen when electrons absorb photons and move to higher energy levels. You will know how spectral lines. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. Created when photons of all wavelengths are emitted. A hot tenuous gas produces a series of brightly coloured lines. Absorption lines are based. Absorption Lines Are Produced By ____.
From www.researchgate.net
Absorption π and σ spectra in the region of 3 H 6 → 3 F 3 transition at Absorption Lines Are Produced By ____ The shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up. Since each atom has its own characteristic set of energy levels, each is associated with. An absorption line at a wavelength of 588 nm is observed in the spectrum from a star. You will be able to distinguish between emission and absorption lines in a spectrum.. Absorption Lines Are Produced By ____.